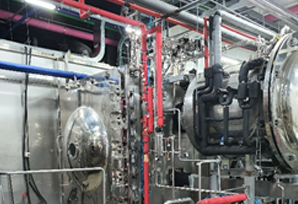
Manufacturing facilities
Manufacturing facilities
meeting global standards
meeting global standards
- 01Implementing strict quality management applying manufacturing and quality management standards suggested by 01PIC/s (Pharmaceutical Inspection Co-operation Scheme)
- 02Establishing hardware and software complying with global regulations such as EU-GMP and cGMP
- 03Building a variety of production lines including tablet, capsule, powder injection, ampoule injection, liquid vial injection, and freeze-dried injection
- 04Manufacturing excellent medicines through formulation and production process technology and strict quality control system







Based on various formulations and excellent quality
We develop and produce customized medicines for various domestic and foreign pharmaceutical companies
We develop and produce customized medicines for various domestic and foreign pharmaceutical companies
-
Contract Development and Manufacturing Organization (CDMO)
Conducting the entire process of developing new drugs and strategic items, including raw material sourcing, development, production process, clinical trials, and commercializing - Contract Manufacturing Organization (CMO) Carrying out the entire process from purchasing raw materials to testing drug products
- Wide Variety of Dosage Forms Possessing various dosage forms (freeze drying, Penem injection, liquid/powder injection, ampoule injection, tablet, capsule, ODT formulation)
- Quality First Satisfying customer expectations by putting quality first
- On Time Delivery Meeting the schedule by optimizing product delivery
- Documentation Service Providing all documents related to CDMO/CMO
93+
Number of companies proceeding CDMO/CMO
(As of March 2025)
318+
Number of items of CDMO/CMO
(As of March 2025)
